Pulse Wave™ 4th Generation Focused Shockwave Therapy Machine | FDA Registered & American-Engineered | Zero Maintenance & Fast ROI
Pulse Wave™ 4th Generation Focused Shockwave Therapy Machine | FDA Registered & American-Engineered | Zero Maintenance & Fast ROI is backordered and will ship as soon as it is back in stock.
Couldn't load pickup availability
Pulse Wave™ 4th Generation Focused Shockwave Therapy Machine | FDA Registered & American-Engineered | Zero Maintenance & Fast ROI
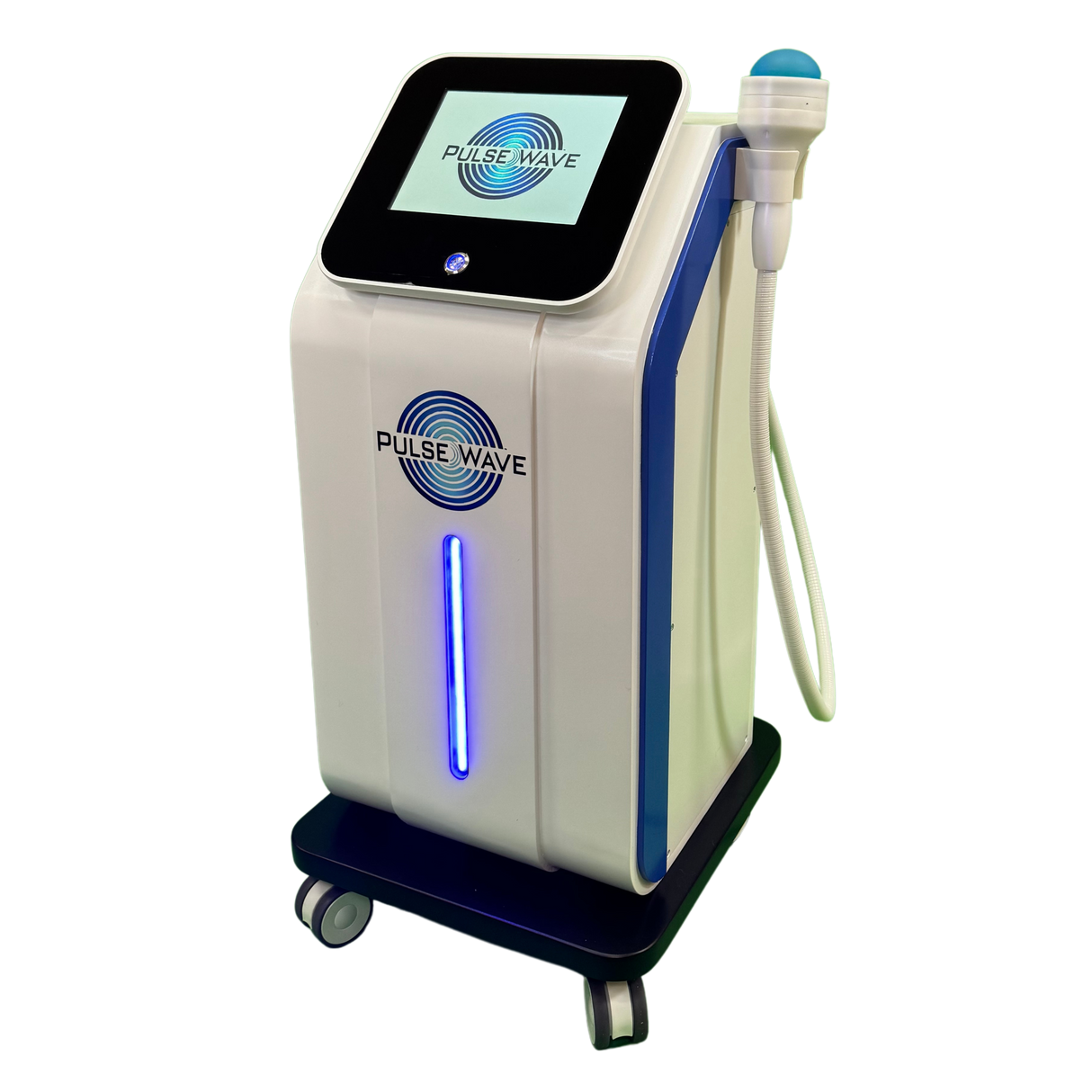
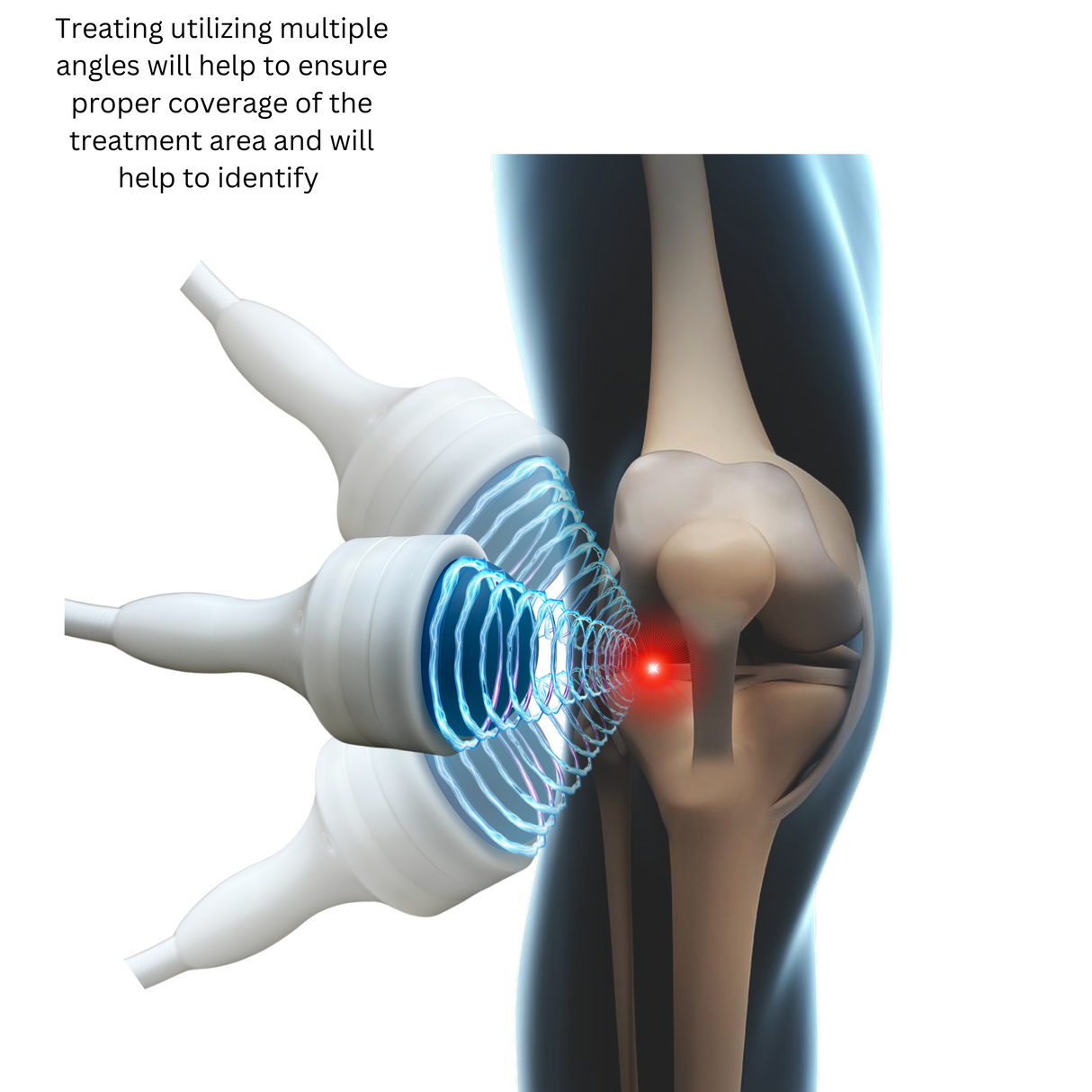
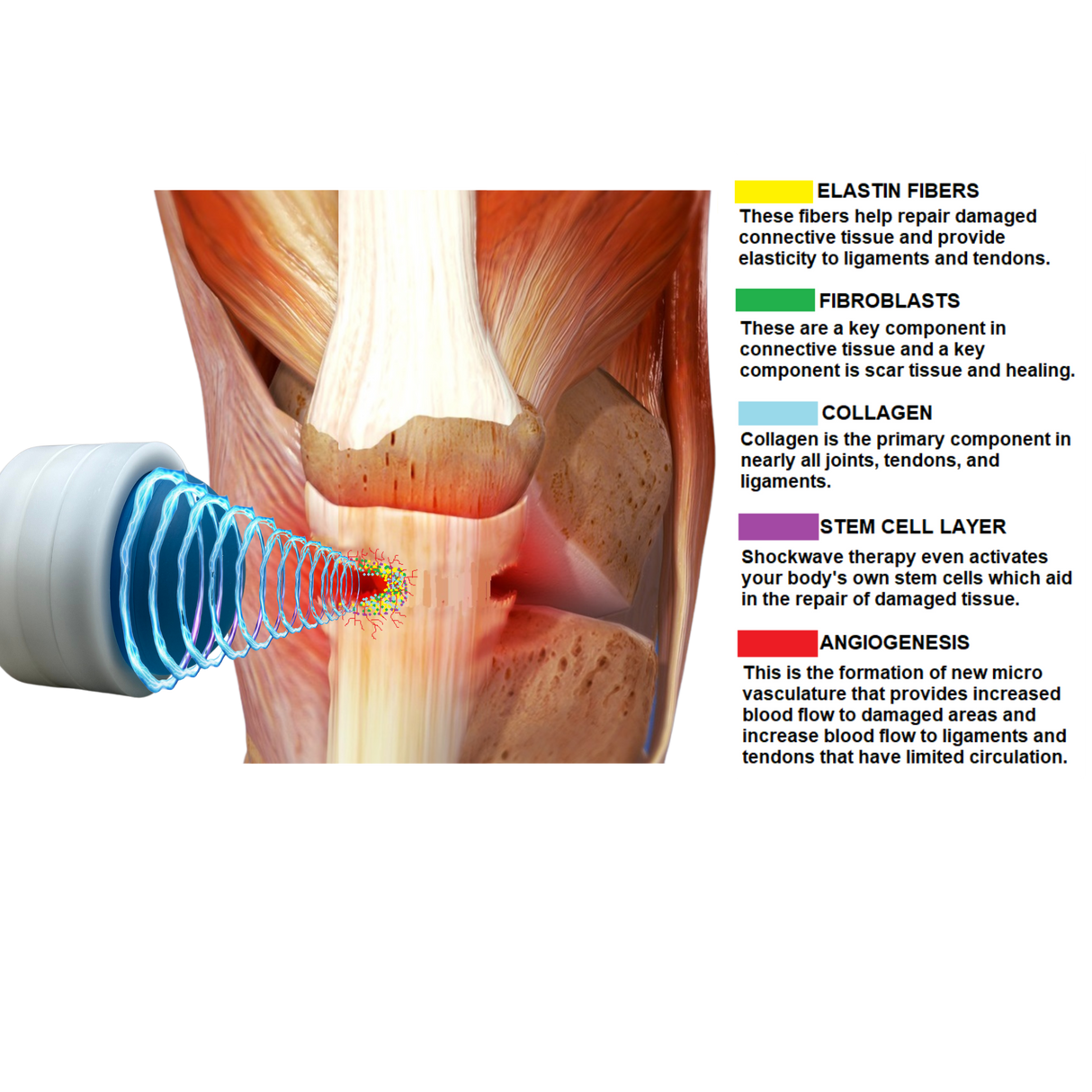
Meet Pulse Wave™—the 4th Generation Focused Shockwave Therapy Machine designed to revolutionize pain management, accelerate recovery, and deliver a rapid return on investment for clinics and sports medicine facilities. Built with advanced electromagnetic technology, Pulse Wave™ provides 100% focused energy to target deeper tissues with minimal discomfort and zero maintenance requirements.
Why Choose Pulse Wave™?
-
American-Engineered & FDA Registered:
Trusted quality and safety, designed by providers for providers. -
Zero Corrosive Saline & Maintenance:
Uses standard sterile water—no more high upkeep or frequent part replacements. -
Rapid ROI & Financing Options:
Priced at $28,990, includes NCMIC financing (60-month plan, up to 90-day deferment), and full ROI often achieved in 4.8 months. -
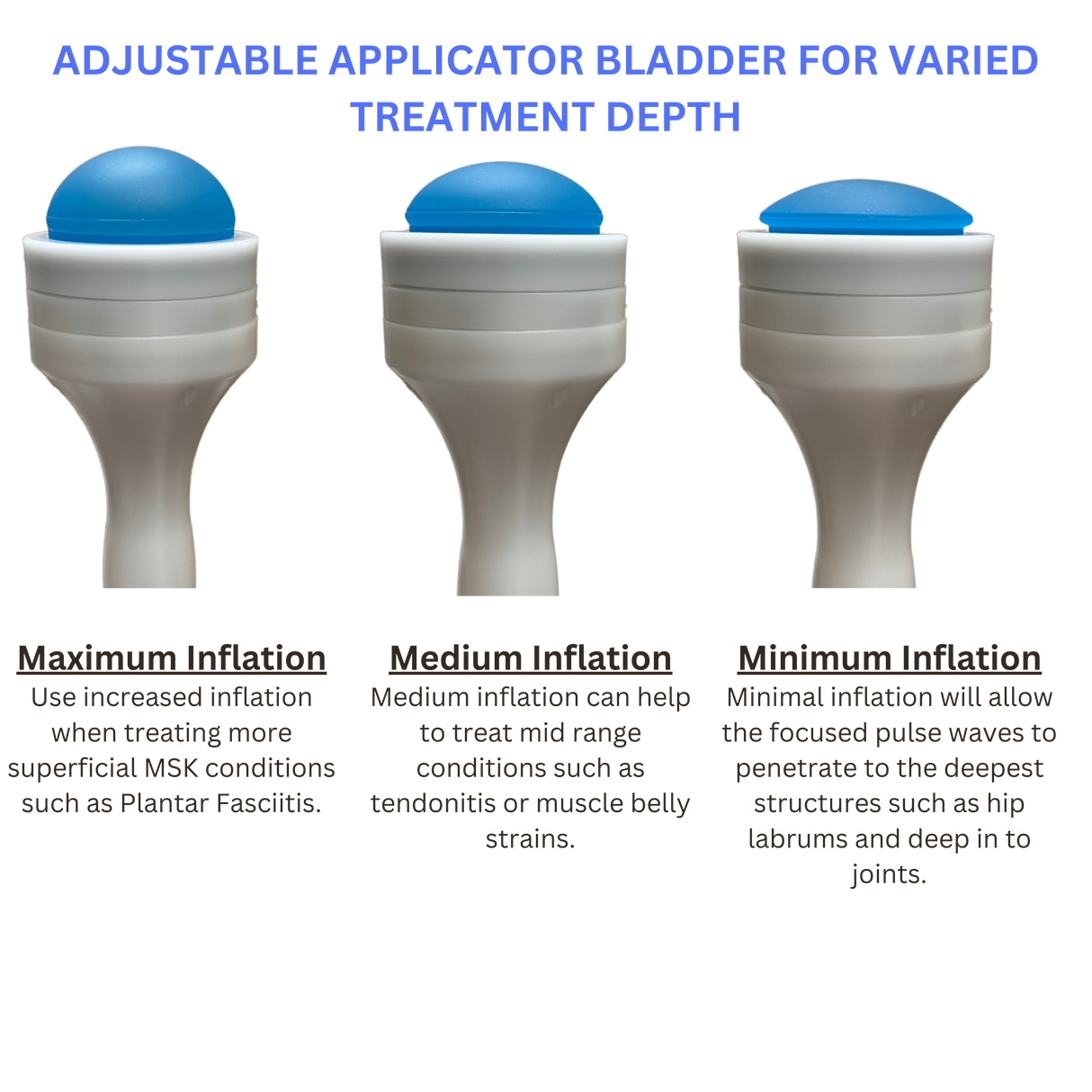
Electromagnetic Precision:
Eliminates spark-gap inefficiencies, delivering consistent pressure for deeper penetration. -
Long Applicator Life:
Up to 1,000,000 pulses—significantly outlasting many outdated systems.
How It Pays for Itself
- Low Monthly Payment
- 5 Patients/Week at $150/Session covers financing
- 10 Patients/Week (~4.8 Months) for full ROI
- 90-Day Deferred Payments = $18,000 profit before first payment
Transform Your Practice Today
Upgrade to the Pulse Wave™ focused shockwave therapy machine and experience the difference of American-engineered, zero-maintenance technology that boosts clinic revenue while enhancing patient outcomes.
Ready to elevate your practice? Contact us for a demo or add to cart now to secure your Pulse Wave™ device and reshape the future of shockwave therapy in your facility!
Discloser:
This device is not cleared for, intended use for, or advertised to treat the heart, brain, skin, liver, or kidney. All major organs are a direct contraindication for the Pulse Wave Device.
This device is not cleared for, intended use for, or advertised to reduce or eliminate a mass such as a calcified plaque, stone, or other. Use of this device is directly contraindicated for such masses or uses.